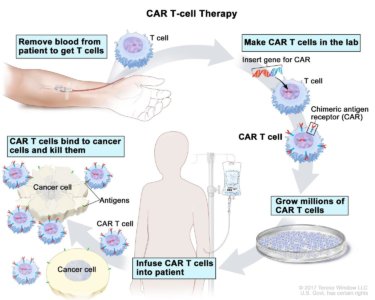
T-cell transfer therapy is a type of immunotherapy that makes your own immune cells better able to attack cancer. There are two main types of T-cell transfer therapy: tumor-infiltrating lymphocytes (or TIL) therapy and chimeric antigen receptor T-cell (CAR T-cell) therapy. Both involve collecting your own immune cells, growing large numbers of these cells in the lab, and then giving the cells back to you through a needle in your vein.
T-cell transfer therapy is also called adoptive cell therapy, adoptive immunotherapy, and immune cell therapy. The process of growing your T cells in the lab can take 2 to 8 weeks. During this time, patients may have treatment with chemotherapy and, maybe, radiation therapy to get rid of other immune cells. Reducing your immune cells helps the transferred T cells to be more effective.
- TIL therapy uses T cells called tumor-infiltrating lymphocytes that are found in your tumor. Doctors test these lymphocytes in the lab to find out which ones best recognize your tumor cells. Then, these selected lymphocytes are treated with substances that make them grow to large numbers quickly. The idea behind this approach is that the lymphocytes that are in or near the tumor have already shown the ability to recognize your tumor cells. But there may not be enough of them to kill the tumor or to overcome the signals that the tumor is releasing to suppress the immune system. Giving you large numbers of the lymphocytes that react best with the tumor can help to overcome these barriers.
- CAR T-cell therapy is similar to TIL therapy, but your T cells are changed in the lab so that they make a type of protein known as CAR before they are grown and given back to you. CAR stands for chimeric antigen receptor. CARs are designed to allow the T cells to attach to specific proteins on the surface of the cancer cells, improving their ability to attack the cancer cells.
SQZ Biotech
One example of an early-stage company pursuing a novel cell therapy is SQZ Biotech with the potential to reduce production time to 24 hours and without pre-conditioning or planned hospitalization Its lead product candidate, SQZ-PBMC-HPV is a targeted cancer vaccine that has been designed to generate antigen-specific CD8+ T cell (CD8, CD8 T cell) responses to attack HPV+ tumors. This week, the company’s Investigational New Drug (IND) application for SQZTM Activating Antigen Carriers (SQZ AACs) in HPV+ tumors was cleared by the U.S. Food and Drug Administration (FDA).
History
T-cell transfer therapy was first studied for the treatment of metastatic melanoma because melanomas often cause a strong immune response and often have many TILs. The use of TIL therapy has been effective for some people with melanoma and has produced promising findings in other cancers, such as cervical squamous cell carcinoma and cholangiocarcinoma. However, this treatment is still experimental.
Three CAR T-cell therapies have been approved by the Food and Drug Administration for blood cancers:
CAR T-cell therapy has also been studied for the treatment of solid tumors, including breast and brain cancers, but use in such cancers is still experimental.
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.