Synthetic Biologics, Inc is clinical-stage company leveraging the microbiome to develop therapeutics designed to treat gastrointestinal (GI) diseases.
The Company’s lead clinical development candidates are: (1) SYN-004 (ribaxamase) which is designed to degrade certain commonly used intravenous (IV) beta-lactam antibiotics within the gastrointestinal (GI) tract to prevent (a) microbiome damage, (b) Clostridioides difficile infection (CDI), (c) overgrowth of pathogenic organisms, (d) the emergence of antimicrobial resistance (AMR) and (e) acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients, and (2) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. .The Company was also developing SYN-010 to reduce the impact of methane-producing organisms in the gut microbiome to treat an underlying cause of irritable bowel syndrome with constipation (IBS-C).
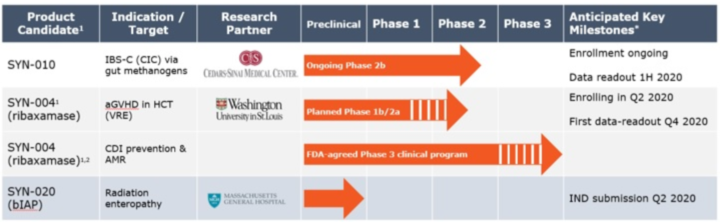
On September 30, 2020, Cedars Sinai Medical Center’s (CSMC) (the Company’s SYN-010 clinical development partner) informed the Company that it agreed to discontinue the ongoing Phase 2b investigator-sponsored clinical study of SYN-010 IBS-C patients. Based on the results of a planned interim futility analysis, it was concluded that although SYN-010 was well tolerated, it was unlikely to meet its primary endpoint by the time enrollment is completed.
As a result of the decision to discontinue the ongoing Phase 2b investigator-sponsored clinical study of SYN-010, the company plans to explore and evaluate a range of strategic options.
SYN-004 (ribaxamase)
Prevention of antibiotic-mediated microbiome damage, C. difficile infections (CDI), overgrowth of pathogenic organisms, the emergence ofantimicrobial resistance (AMR) and acute graft-versus-host disease (aGVHD) in allogeneic HCT recipientsSYN-004 (ribaxamase) is a proprietary oral capsule prophylactic therapy designed to degrade certain IV beta-lactam antibiotics excreted into the GI tract and thereby maintain the natural balance of the gut microbiome.
Preventing beta-lactam damage to the gut microbiome has a range of potential therapeutic outcomes, including prevention of CDI, suppression of the overgrowth of pathogenic species (particularly antimicrobial-resistant organisms) and potentially reducing the incidence of acute graft-versus-host-disease(aGVHD) in allogeneic hematopoietic cell transplant (HCT) patients. SYN-004 (ribaxamase) is a beta-lactamase enzyme intended to be co-administered with certain IV beta-lactam antibiotics as two-75 mg capsules which, when released in the proximal small intestine, has been shown to degrade beta-lactam antibiotics in the GI tract without alteringsystemic antibiotic levels. Beta-lactam antibiotics are a mainstay in hospital infection management and include the commonly used penicillin and cephalosporin classes ofantibiotics.C. difficile InfectionC. difficile is the leading type of hospital acquired infection and is frequently associated with IV beta-lactam antibiotic treatment.
The CDC identified C. difficile as an “urgent public health threat,” particularly given its resistance to many drugs used to treat other infections. CDI is a major unintended risk associated with the prophylactic or therapeutic use of IV antibiotics, which may adversely alter the natural balance of microflora that normally protect the GI tract, leading to C. difficile overgrowth and infection. Other risk factors for CDI include hospitalization, prolonged length of stay (estimated at 7 days), underlying illness, and immune-compromising conditions including the administration of chemotherapy and advanced age.
According to a paper published in BMC Infectious Diseases (Desai K et al. BMC Infect Dis. 2016; 16: 303) the economic cost of CDI was approximately $5.4 billion in 2016 ($4.7 billion in healthcare settings; $725 million in the community) in the U.S., mostly due to hospitalizations.
CDI is a widespread and often drug resistant infectious disease. Approximately 20% of patients who have been diagnosed with CDI experience a recurrence of CDI within oneto three months. Furthermore, controlling the spread of CDI has proven challenging, as the C. difficile spores are easily transferred to patients via normal contact with healthcare personnel and with inanimate objects.
There is currently no vaccine or approved product for the prevention of primary (incident) CDI. The current standard of care for primary CDI, as outlined by the Infectious Disease Society of America (IDSA), is to treat with powerful antibiotics such as fidaxomicin or vancomycin. Prolonged use of fidaxomicin and vancomycin has been shown to further exacerbate damage to the gut microbiome, leading to increased risk of CDI recurrence as well as the emergence of pathogenic and antimicrobial-resistant (AMR) organisms, such as vancomycin-resistant enterococci (VRE).
AMR is a serious global threat and one which world leaders have begun to takeaction against. According to the European Society of Clinical Microbiology and Infections Disease (ECCMID), failure to address AMR could lead to a potential antibiotic Armageddon, resulting in 10 million deaths worldwide by 2050 and may cost as much as $100 trillion in worldwide economic output.