Sensei Biotherapeutics, Inc., a Phase 2 biotech company based in Rockville, MD, is a developing bacteriophage-based platform for cancer therapies. In the first week of February, the company raised $133 million by offering 7 million shares at $19, above the range of $16 to $18.
Sensei’s ImmunoPhage platform uses bacteriophage to induce an innate and adaptive immune response. The company’s lead candidate, SNS-301, is currently in an ongoing 30-patient Phase 1/2 trial in combination with the PD-1 inhibitor pembrolizumab for squamous cell carcinoma of the head and neck. Topline data is expected by the end of 2021, and if the results are positive, subject to feedback from the FDA, the company intends to initiate a randomized, registration-enabling trial.
The Problem
According to Sensei, one of the most promising classes of therapeutics for the treatment of cancer is monoclonal antibodies that target programmed cell death protein 1, or PD-1, and its related ligand, or PD-L1. However, in a majority of patients they generally fail to produce meaningful results. Drugs using PD-1 blockade have been approved by the FDA to treat at least 20 different types of cancer and, in 2019, generated sales of approximately $19.4 billion worldwide. By 2024, the total global market for drugs using PD-1 blockade is estimated to exceed $36 billion, according to Sensei. Two of the most common reasons for non-response to PD-1 blockade treatment include a lack of tumor infiltrating lymphocytes, or TILs, or the presence of alternate immunosuppressive mechanisms such as VISTA. There has been considerable focus on the development of therapies that induce the body’s immune system to mount a response towards tumor antigen targets to address these mechanisms of non-response to PD-1 blockade.
The Solution
Sensei’s ImmunoPhage platform is designed to address the challenges of converting PD-1 blockade non-responsive tumors into responsive ones by triggering the generation of tumor antigen-specific T cells and circumventing immunosuppressive pathways.
Promising work with bacteriophage led to Sensei’s discovery of their utility as a powerful, self-adjuvanted immunotherapy platform. The foundation of ImmunoPhage is the bacteriophage lambda, or lambda phage, which the company selected for its native immunostimulatory capabilities, large and tractable genome, and tolerability profile.
The highly immunogenic nature of bacteriophage promotes a balanced, coordinated and robust response by both the innate and the cellular and humoral components of the adaptive immune system. The company believes that the unique features of bacteriophage, including the ability to generate both T cell responses and B cell mediated antibody responses, give it the potential to be used in the development of differentiated treatments for cancer.
The modularity of the ImmunoPhage platform allows for personalized, dynamic substitution of particular phage components to optimize patient therapy. Sensei’s tactic is to enhance the use, precision and therapeutic activity of its product candidates with phage cocktails that express multivalent antigens along with the integration of nanobody technology. This allows for an adaptive clinical trial design, which is under discussion with the FDA. To date, Sensei has constructed over 25 unique ImmunoPhage configurations in-house in accordance with current good manufacturing practices, or cGMP, and the company continues to expand its Phortress library of ImmunoPhages.
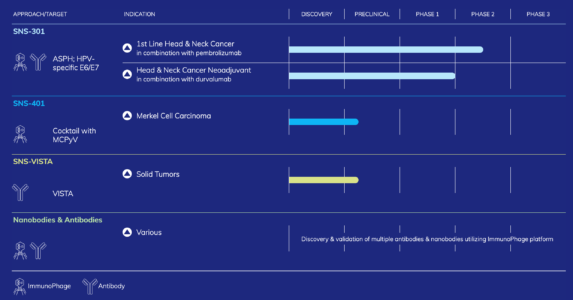
SNS-301
Early data with SNS-301 as a monotherapy and in combination with immune checkpoint inhibitors have demonstrated that it has generally been well tolerated, shows improvements in disease biomarkers and has the potential to generate a robust, dose-dependent antigen-specific CD8+ T cell and B-cell response. Sensei is currently conducting a Phase 1/2 trial of SNS-301 in combination with Keytruda™ (pembrolizumab) in squamous cell carcinoma of the head and neck (SCCHN) cancer patients who did not achieve tumor reductions on anti-PD-1/PD-L1 therapy alone (NCT04034225). SCCHN cancer is the sixth most common malignancy worldwide, accounting for approximately 6% of all cancer cases, and is responsible for an estimated 1% to 2% of all cancer deaths. An estimated 650,000 cases of head and neck cancer are diagnosed annually worldwide, including approximately 50,000 cases in the United States.
Human papilloma virus, or HPV, infection accounts for an estimated 70% of SCCHN cases in the U.S.. The current standard of care in the company’s target patient population is PD-1 inhibition as a single agent or in combination with chemotherapy. Despite improvements in diagnoses and disease management, long-term survival rates for patients with SCCHN have not increased significantly over the past 30 years and are among the lowest for major cancers. The company selected SCCHN as our first indication based on a high unmet patient need, robust scientific rationale, a clearly defined regulatory path and accessibility of these tumors for biopsy. SNS-301 has been engineered to produce a targeted immune response against the tumor associated antigen, or TAA, aspartate b-hydroxylase, or ASPH. ASPH is found to be overexpressed in 70% to 90% of human malignancies, including SCCHN. Expression of ASPH is related to cancer cell growth, invasiveness, and disease progression through the Notch signaling pathway.
Sensei believes SCCHN tumors are often lacking intratumoral CD8 T cells and as such the addition of SNS-301 has the potential to generate and expand ASPH specific anti-tumor T cells and thereby enhance PD-1 blockade activity. The company is currently evaluating SNS-301 in combination with the PD-1 inhibitor pembrolizumab in a 30-patient Phase 1/2 clinical trial.
As of December 10, 2020, Sensei has enrolled 11 patients in the trial, of which ten patients were evaluable for efficacy. The trial includes patients with locally advanced unresectable or metastatic SCCHN who have been treated with PD-1 blockade for at least 12 weeks with the best overall response being stable disease (SD) or unconfirmed progressive disease, or PD. Patients who achieved a partial response (PR), complete response, or CR, or confirmed progression on PD-1 blockade, are not eligible. Based on an initial assessment of the ten evaluable patients, SNS-301 in combination with pembrolizumab has been well tolerated and has shown promising anti-tumor activity, including a PR in one patient with a PD-L1 negative tumor who achieved SD as best overall response on PD-1 inhibition alone as well as SD in six patients.
Of the six SD patients, one patient previously had PD on PD-L1 inhibition and two patients have achieved longstanding SD for greater than 36 weeks following treatment. The company anticipates reporting topline data from this trial by the end of 2021. If the results of this trial are positive, subject to feedback from the FDA, the company intends to initiate a randomized, registration-enabling trial for SNS-301.
The company also intends to evaluate the addition of SNS-301 to pembrolizumab in PD-1 blockade naïve SCCHN patients as part of its ongoing Phase 1/2 trial, with enrollment in this additional treatment arm expected to begin in mid-2021. Sensei intends to use an ImmunoPhage cocktail targeting the E6/E7 antigens of HPV, in combination with SNS-301, in HPV positive patients in its ongoing trial of SNS-301, which it expect to incorporate in mid-2021.
In addition, the company is currently planning two Phase 2 trials to evaluate the safety and efficacy of SNS-301 in combination with durvalumab for patients with locally advanced resectable SCCHN in the neoadjuvant setting and ASPH positive patients with locally advanced unresectable or metastatic solid tumors. Sensei intend to initiate the first trial in patients with locally advanced resectable SCCHN in the neoadjuvant setting in mid-2021.
In addition to SNS-301, the company is currently developing its next ImmunoPhage candidate, SNS-401, for the treatment of Merkel cell carcinoma (MCC). SNS-401 is in preclinical studies, and the company plans to submit an investigational new drug application, or IND, for SNS-401 in the first half of 2022. Sensei is also developing a mAb therapy targeting V-set immunoglobulin domain suppressor of T cell activation (VISTA). Through the use of proprietary functional and in vivo assays, the company intends to select a product candidate and initiate IND-enabling studies for our lead mAb by the end of 2021.
Beyond the current therapies under development, the company envisions finding cancer through early detection techniques such as liquid biopsy and training its ImmunoPhage platform to knock out cancer before it develops past stage one or two.
Source: Sensei Biotherapeutics, Inc
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.

