Recursion Pharmaceuticals Inc. uses machine learning to hunt for new therapies. The company raised $436 million in an upsized initial public offering priced at the top of a marketed range.
Last week , the Salt Lake City-based company sold about 24 million shares for $18 each.
Recursion is among a crop of new startups called digital native companies in the drug discovery business. According to Lina Nilsson, Recursion’s Vice President of Product, “we have a unified, company-wide platform with common coding languages, data storage frameworks and tools. Digital natives live fully in today’s integrated software world, without large networks of incompatible legacy systems.” Lina also comments that digital native biotech startups are already balancing and integrating the worlds of tech (e.g. engineering and data science) and science (e.g. biology and chemistry) and as such have an edge against larger companies with vastly more resources than Recursion. Yet the static or declining level of R&D output at many large companies means that they have an ongoing need for new projects to fill their pipelines.
The company develops its technical infrastructure as interlocking products, borrowing from tech’s’ use of product management to align across business, technology and science disciplines to crystallize out the most impactful areas of focus. It’s all about industrialized drug discovery. Lina says, “we develop our technical infrastructure as interlocking products, borrowing from tech’s’ use of product management to align across business, technology and science disciplines to crystallize out the most impactful areas of focus. We don’t just build new data science models, we create the frameworks to build the right models and build rigorous infrastructure to implement at scale. Rather than individual tools, the focus is on systems of products.”
Recursion reached a deal with Bayer AG in September 2020 to use AI to find new drugs for lung fibrosis and other fibrotic diseases. The deal included a $30 million upfront payment and $50 million in equity funding by Bayer’s investment arm.
Pipeline
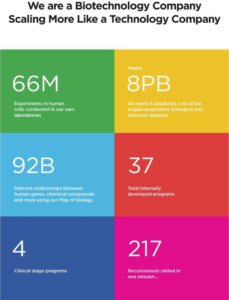
The company is advancing 37 programs, and there are ten ‘Notable Programs’ that are key, near-term value drivers given their individual market opportunities and the validation they provide for each generation of the Recursion OS, the company’s platform software that integrates a multi-layer system for generating, analyzing, and deriving insights from biological and chemical datasets.
Brute-Force Search Programs
Eight of Recursion’s Notable Programs were identified using what the company calls brute-force search approach. Four of these programs are new uses of existing known chemical entities, or KCEs, that that company has advanced to clinical development and for which they have obtained key enabling licenses. Another four of these programs are new chemical entities, or NCEs, that have been discovered and advanced in-house.
- REC-4881 for the Treatment of FAP. REC-4881 is an orally bioavailable, non-ATP-competitive allosteric small moleculeinhibitor of MEK1 and MEK2 being developed to reduce tumor size in familial adenomatous polyposis, or FAP, patients. REC-4881 appears to be well tolerated, consistent with the intended use and a gut-localized PK profile in humans that is highly advantageous for FAP and potentially other tumors of the gastrointestinal tract. The company expects to enroll the first patient in a Phase 2, double-blind, randomized, placebo-controlled trial within the next four to five quarters.
- REC-3599 for the Treatment of GM2 Gangliosidosis. REC-3599 is an orally bioavailable, selective, potent smallmolecule inhibitor of protein kinase C, or PKC, and glycogen synthase kinase 3 beta, or GSK3ß, being developed for the treatment of GM2 gangliosidosis. This molecule has demonstrated strong reduction of pathogenic biomarkers GM2 and lipofuscin levels in cells derived from patients with multiple different mutations in either HEXA or HEXB, referred to as Tay-Sachs or Sandhoff Disease, respectively. The company currently generating additional pharmacodynamic data in a HEXB-mutant animal model of GM2 at the request of the FDA in anticipation of enrolling the first patient in an open-label Phase 2 trial within the next four to five quarters.
- REC-2282 for the Treatment of NF2. REC-2282 is a CNS-penetrant, orally bioavailable, small molecule histonedeacetylase, or HDAC, inhibitor being developed for the treatment of NF2-driven meningioma and neurofibromatosis type 2. This molecule appears to be well tolerated, including in patients dosed for multiple years, and potentially has reduced cardiac toxicity that would differentiate it from other HDAC inhibitors. Its oral bioavailability and CNS penetrance distinguish it from currently approved HDAC inhibitors. The company expect to enroll the first patient in an adaptive, parallel group, Phase 2/3, randomized, multicenter study within the next four to five quarters.
- REC-994 for the Treatment of CCM. REC-994 is an orally bioavailable superoxide scavenger small molecule beingdeveloped for the treatment of cerebral cavernous malformations, or CCM. In Phase 1 single-ascending dose, or SAD, and multiple-ascending dose, or MAD, trials in healthy volunteers that we conducted, REC-994 demonstrated tolerability and suitability for chronic dosing. CCM is among the largest areas of unmet need in rare disease, affecting approximately 360,000 symptomatic patients in the United States and EU5, and has no approved therapies. The company expects to enroll the first patient in a Phase 2, double-blind, placebo-controlled, safety, tolerability and exploratory efficacy study within the next four to five quarters.
- Lead Molecules for the Treatment of C. difficile Colitis. We have identified three lead NCEs (REC-163964,REC-164014, and REC-164067) with the potential to be orally active, gut-biased, small molecule C. difficile toxin inhibitors, which the company has shown to act via inhibition of glucosyl transferase. These molecules have the potential to prevent recurrent disease and be used as secondary prophylaxis therapy in high-risk patients with C. difficile infections, the leading cause of antibiotic-associated diarrhea and a major cause of morbidity and mortality. The company is currently completing exploratory non-clinical safety studies to enable potential selection of a development candidate.
- Lead Molecules for the Treatment of Neuroinflammation. We have identified three lead NCEs (REC-648455,REC-648597, and REC-648677) with the potential to be orally bioavailable, safe, CNS-penetrant, small molecule modulators of microglial activation. Microglial activation and neuroinflammation are hallmarks of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, and CNS inflammatory diseases such as multiple sclerosis. Small molecule modulators of microglial activation have the potential to reduce neuronal death associated with proinflammatory processes in neurodegenerative diseases and inflammatory diseases of the CNS. The project is in lead optimization.
- Lead Molecules for the Treatment of Batten Disease. The company has identified three lead NCEs (REC-648190, REC-259618,and REC-648647) with the potential to be orally bioavailable, CNS-penetrant, disease modifying therapeutics for multiple subtypes of Batten disease. Batten disease is an autosomal recessive, neurodegenerative disease resulting from mutations in one of fourteen CLN genes. While rare, these disorders collectively represent the most prevalent pediatric neurodegenerative disease and demonstrate significant unmet need. This project is currently in lead optimization.
- Lead Molecules for the Treatment of CMT2A. The company has identified four lead molecules (REC-64810, REC-648458,REC-1262, and REC-150357) with the potential to be orally bioavailable, disease modifying molecules to slow or reverse the progression of the mitochondrial disease Charcot-Marie-Tooth type 2A, or CMT2A. CMT2A is a rare, autosomal dominant, peripheral nerve degenerative disease caused by mutations in the MFN2 gene which leads to progressive muscle atrophy in the lower legs and hands. There are no approved disease modifying therapies for CMT2A. This project is currently in lead optimization.
Inferential Search Programs
Two of Recursion’s Notable Programs were identified since mid-2020 using its new inferential search approach. One of these programs is a new use of an existing KCE while the other is an NCE discovered and advanced in-house. The speed with which the company has been able to identify and initiate early discoveries with inferential search programs demonstrates the potential power of the Recursion OS to generate high-quality hits to move through the lead optimization process.
- REC-64151 for the Treatment of Immune Checkpoint Resistance in STK11-mutant NSCLC. The company has identified a novel potential use for a clinical-stage, orally bioavailable small molecule to restore and improve sensitivity to immune checkpoint inhibitors in tumors harboring mutations in the tumor suppressor gene STK11. There are approximately 30,000 cases per year of STK11-mutant metastatic non-small cell lung cancer, or NSCLC, in the US and EU5, and these mutations have been shown to predict poor prognosis and resistance to immune checkpoint inhibitors, or ICI, specifically anti-PD-(L)1 therapies. There are currently no approved therapies developed to specifically modulate tumor response in STK11-mutant cancers. This program is currently in the dose-optimization phase.
- MYC-Inhibitory Molecules for the Treatment of Solid and Hematological Malignancies. The company has identified multiple hit series using its inferential-search approach that have subsequently shown concentration-dependent activity in suppressing transcriptional activity downstream of MYC. Increased expression of MYC transcriptional target genes presents across oncology and up to 50% of cancers harbor alterations in MYC. Novel small molecules with the potential to suppress MYC-dependent activity could improve treatment of diverse tumors, especially those harboring mutations in genes directly implicated in MYC activation. There are currently no approved molecules that target MYC specifically. This program is currently in the hit-to-lead phase.
In addition to the Notable Programs highlighted above, the company is actively exploring 27 additional programs which may prove to be drivers of our future growth. Of these programs, 10 arose out of our brute-force search approach, and 17 were identified using our newer inferential search approach since July 2020.
Moving forward, the company expects that the vast majority of its new programs will be discovered using its inferential search approach. The company believes that the number of potential programs it can generate with its Recursion OS is key to the future of our company, as a greater volume of validated programs has a higher likelihood of creating value. The speed at which its Recursion OS generates a large number of product candidates is important, since traditional drug development often takes a decade or more. In addition, the company believes that our large number of potential programs makes it an attractive partner for larger pharmaceutical companies.
Source: Recursion Pharmaceuticals Inc
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.