Caribou Biosciences is a clinical-stage biopharmaceutical company innovating in next-generation, genome-edited cell therapies. Its CRISPR hybrid RNA-DNA (chRDNA) guide technology has demonstrated superior specificity and high efficiency in preclinical studies, and, according to the company, has the potential to generate gene and cell therapies in oncology and in therapeutic areas beyond oncology, including immune cell therapies, cell therapies derived from genome-edited iPSCs, and in vivo genome-editing therapies.
Caribou was founded in 2011 by leaders in CRISPR and nucleic acid biology: Jennifer A. Doudna, Ph.D., who was a co-recipient of the 2020 Nobel Prize in Chemistry for the development of CRISPR-Cas9 as a method for genome editing; Martin Jinek, Ph.D., Assistant Professor at the University of Zurich in the Department of Biochemistry; James Berger, Ph.D., Professor in the Department of Biophysics and Biophysical Chemistry at the Johns Hopkins University School of Medicine; and Rachel E. Haurwitz, Ph.D., who has served as our President and Chief Executive Officer since our founding.
Since its founding, Caribou has raised approximately $150.1 million in net proceeds from equity capital invested by leading venture capital funds, healthcare-dedicated funds, other institutional investors, and strategic investors. Its institutional investors include Adage Capital Partners, Anterra F&A Ventures, Avego Bioscience Capital, Avidity Partners, Invus, a fund affiliated with Farallon Capital Management, F-Prime Capital Partners Healthcare Fund IV LP, Heritage Medical Systems, Janus Henderson Investors, LifeSci Venture Partners, Maverick funds, Mission Bay Capital, Monashee Investment Management, funds affiliated with PFM Health Sciences, Point72, Ridgeback Capital Investments, Pontifax Global Food and Agriculture Technology Fund, and funds managed by Tekla Capital Management.
Corporate and strategic investors include AbbVie, DuPont, Genus, The Leukemia & Lymphoma Society Therapy Acceleration Program, and Novartis.
Additionally, Caribou has received approximately $161.1 million from various licensing, collaboration, patent assignment, and service agreements as well as government grants, including approximately $88.4 million in net proceeds from the sale of Intellia Therapeutics, Inc., or Intellia, common stock received as consideration for our CRISPR-Cas9 license agreement with Intellia, and $30.0 million received from AbbVie as an upfront payment for its collaboration and license agreement with AbbVie.
In total, the company has received a total of approximately $311.2 million in net proceeds from equity financings and contract revenues.
Current Challenges in Allogeneic Cell Therapies
According to the company, immune cell therapies have emerged as a revolutionary and potentially curative treatment for hematologic malignancies and solid tumors. The approval and launch of multiple first generation CD19- or BCMA-directed autologous CAR-T cell products have laid the foundation and opened a path for the development of more advanced cell therapeutics, including CAR-T and CAR-NK cell products with next-generation capabilities and approaches. Among these approaches, allogeneic cell therapy is positioned to unlock the broad potential of engineered immune cells as a leading therapeutic modality. However, expansion, persistence, and trafficking of allogeneic CAR-T and CAR-NK cells are critical to achieving long-term efficacy. Caribou intends to capitalize on current deficiencies in the genome-editing technologies currently used in the allogeneic cell therapy arena.
Pipeline
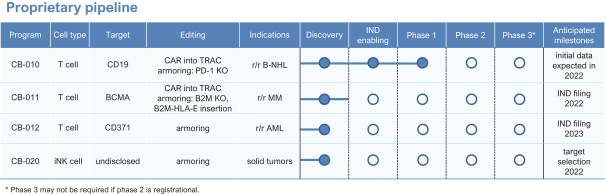
The company has been focused on advancing multiple proprietary allogeneic cell therapies for the treatment of both hematologic malignancies and solid tumors against cell surface targets for which autologous CAR-T cell therapeutics have previously demonstrated clinical proof of concept, including both CD19 and BCMA, as well as new targets. Caribou uses its chRDNA technology to enhance, or armor, cell therapies by creating additional genomic edits to improve persistence of antitumor activity.
Intellectual Property
Caribou has invented and acquired broad intellectual property covering chRDNA genome editing as well as additional genome-editing and cell therapy technologies. Currently, its owns 48 issued U.S. patents, including 7 U.S. patents covering our chRDNA technology; 209 issued foreign patents; and 93 pending patent applications throughout the world.
Its patent portfolio includes granted U.S. patents covering methods and compositions relating to the anti-BCMA binding domain of our CB-011 product candidate. Caribou has exclusively in-licensed intellectual property covering the anti-CD371 binding domains of its CB-012 product candidate from Memorial Sloan Kettering Cancer Center, or MSKCC. Additionally, the company has extensive patent protection on CRISPR Type I systems, CRISPR-Cas9 methods and compositions, and other genome-editing technologies. Without any patent term extension, the earliest expiration dates of its granted U.S. patents are in 2032 and the latest expiration dates of our granted U.S. patents are in 2040.
The company also relies on trade secrets to protect aspects of its manufacturing that are not amenable to patent protection or infringement detection. Under an exclusive license agreement with The Regents of the University of California, or UC, and the University of Vienna, or Vienna, Caribou has a worldwide license, with the right to sublicense, in all fields to the foundational CRISPR-Cas9 patent family co-owned by UC, Vienna, and Dr. Emmanuelle Charpentier, or the CVC IP.
To date, the company has entered into over 20 sublicensing agreements with third parties under which it has granted rights to the CVC IP and other Cas9 intellectual property owned or controlled by Caribou in a variety of fields such as human therapeutics, agriculture, research reagents, transgenic animals, certain livestock targets, internal research, bioproduction, cell lines, microbial applications, and forestry.
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.