Immunotherapy is a type of cancer treatment that helps your immune system fight cancer. The immune system helps your body fight infections and other diseases. It is made up of white blood cells and organs and tissues of the lymph system. Cancer immunotherapy is sometimes referred to as immuno-oncology.
Immunotherapy is a type of biological therapy. Biological therapy is a type of treatment that uses substances made from living organisms to treat cancer. Today, The FDA has approved nearly 40 cancer immunotherapies.
According to Cypherbio, some notable milestones in the immuno-oncology field include the first cancer vaccine, Dendreon Pharmaceuticals’ Sipuleucel T for prostate cancer, approved in 2010; the first checkpoint inhibitor, BMS’ Ipilimumab for metastatic melanoma, approved in 2011; the first oncolytic virus therapy, Amgen’s T-Vec for melanoma, approved in 2015; and the first CAR-T cell therapy, Novartis’ Tisagenlecleucel for acute lymphoblastic leukemia, approved in 2017.
Several types of immunotherapy are used to treat cancer. These include:
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.
- Immune checkpoint inhibitors, which are drugs that block immune checkpoints. These checkpoints are a normal part of the immune system and keep immune responses from being too strong. By blocking them, these drugs allow immune cells to respond more strongly to cancer.
- T-cell transfer therapy, which is a treatment that boosts the natural ability of your T cells to fight cancer. In this treatment, immune cells are taken from your tumor. Those that are most active against your cancer are selected or changed in the lab to better attack your cancer cells, grown in large batches, and put back into your body through a needle in a vein. T-cell transfer therapy may also be called adoptive cell therapy, adoptive immunotherapy, or immune cell therapy.
- Monoclonal antibodies, which are immune system proteins created in the lab that are designed to bind to specific targets on cancer cells. Some monoclonal antibodies mark cancer cells so that they will be better seen and destroyed by the immune system. Such monoclonal antibodies are a type of immunotherapy. Monoclonal antibodies may also be called therapeutic antibodies.
- Treatment vaccines, which work against cancer by boosting your immune system’s response to cancer cells. Treatment vaccines are different from the ones that help prevent disease.
- Immune system modulators, which enhance the body’s immune response against cancer. Some of these agents affect specific parts of the immune system, whereas others affect the immune system in a more general way.

Antibody Therapies
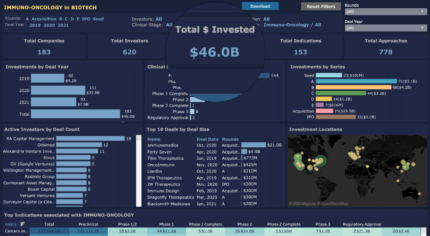
In addition to Immunomedics, 45 other companies are using antibody-based immunotherapies. They received $32.3B, making antibody therapies the top approach and most common immunotherapy method. Furthest along in clinical trials are Immunmedics (approved Trodelvy) and 2 companies that have product candidates in phase 2. Apexigen Inc. developed Sotigalimab, a CD40 agonist monoclonal antibody currently in phase 2 for soft tissue sarcoma that recently was granted Orphan Drug Designation from the FDA. Apexigen raised a $65 Million Series C extension round in March 2020 led by Decheng Capital and Ocean Pine Capital. NextCure has developed NC318, a monoclonal antibody targeting Siglec-15 (S15) for treatment of S15 expressing cancers including non-small cell lung cancer, head and neck squamous cell carcinoma, ovarian and triple-negative breast cancers. NextCure went public in an $86.2 Million IPO in May 2019 valued at $329 Million with a current valuation of $198.8 Million. The majority of the antibody therapy companies, 35 of the 45, have products in preclinical trials. Despite not yet having clinical product candidates, Pyxis Oncology had the 2nd biggest investment round of all antibody therapy companies and the biggest of the preclinical antibody therapy companies. In March 2021, Boston-based Pyxis raised a $152 Million Series B led by Arix Bioscience and RTW Investments to support their preclinical work with antibody-drug conjugate and monoclonal antibody immunotherapies for cancer treatment.Cell Therapies
Cell-based therapies are the second top immuno-oncology method with 41 companies raising $4.7 Billion. Recent advancements and promising results in cancer cell therapy are predominantly due to CAR-T and other t-cell therapies. 29 of the 41 cell therapy companies are using t-cells and 15 of those are using CAR-T cells. However, immuno-oncology cell therapy company Dragonfly Therapeutics is reeling in big funds and does not use the popular t-cells or CAR-T cells. Waltham, MA-based Dragonfly Therapeutics’ platform uses natural killer (NK) cells to treat solid and heme tumors, with their lead NK product candidate in phase 1 trials for HER2 expressing solid tumors and an additional cytokine therapy in phase 1/2 trials. Although the amount of Dragonfly’s most recent investment round in March 2020 was undisclosed, the round brought the company’s total equity to $300 Million, and since then the company has gained massive funds from a $475 Million partnership with BMS and an expansion of a pre-established partnership worth $700 Million with Merck. Merck partnered with another NK cell company, Artiva Biotherapeutics, to help advance Artiva’s pipeline of CAR-NK immunotherapies. Since its founding at the end of 2018, Artiva raised a $78 Million Series A in June 2020 and went on to raise $120 Million Series B in February 2021 led by Venrock Healthcare Capital Partners. The company’s lead product candidate has already begun clinical testing in a phase 1/2 trial for patients with relapsed B-cell non-Hodgkin lymphoma (NHL).IPOs
35 companies went public from 2019-2021 through deals totaling $5 Billion. 30+ of the companies have products in clinical trials including two in phase 3 trials. CStone Pharmaceuticals, which went public in February 2019 through a $285 Million IPO, is the only company with FDA-approved products. 24 of the companies that went public are still preclinical. One of these companies is Cambridge MA-based Werewolf Therapeutics that is advancing its pipeline of proinflammatory immune-modulating cytokines for cancer treatment. Two years after being founded in 2017, Werewolf came out of stealth mode with a $56 Million Series A in November 2019 followed by a $72 Million Series B in January 2021 led by RA Capital Management. Just three months later Werewolf went public with a $120 Million IPO valued at $440 Million to advance their products into IND filing. A later-stage company, California based ALX Oncology went public in July 2020 with a valuation of $668.8 Million, and just over a year later they have a current valuation of 2.64 Billion. ALX is advancing its CD47 checkpoint inhibitor, ALX148, through a number of clinical trials for a series of cancer indications. 2021 to date has seen 93 immuno-oncology deals totaling $7.9B, on par to compete with 111 deals in 2020. Additionally, nearly 38 companies have products in phase 2 trials or beyond, with hopes of being the next major treatment in the immuno-oncology field. Source: CipherbioThe ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.
Immuno-oncology Companies Raised $46 Billion In Recent Years was last modified: September 23rd, 2021 by

