Verve Therapeutics, a biotech company innovating new approaches to the care of cardiovascular disease with single-course gene editing medicines, announced terms for its $201 million IPO on today.
Insiders Wellington Management and Casdin Capital and new investor Fidelity have indicated an interest in purchasing up to an aggregate $75 million worth of shares in the offering. At the midpoint of the proposed range, Verve Therapeutics, founded in 2018, would have a fully diluted market value of $812 million. Like many of the most promising synesthetic biology companies, its youth is not a liability as the tools for innovating with synthetic biology have only just emerged in the last five or so years.
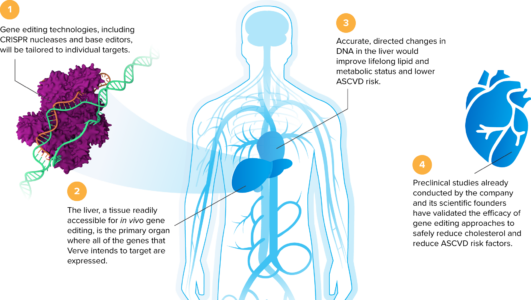
Verve is advancing a pipeline of single-course in vivo gene editing programs, each designed to mimic natural disease resistance mutations and turn off specific genes in order to lower blood lipids.
The company last month announced proof-of-concept data highlighting the company’s gene editing approach for the treatment of cardiovascular disease in the journal Nature. Data reported included that from an ongoing preclinical study of the administration of a single gene editing treatment in non-human primates (NHPs). In this study, Verve observed potent and durable lowering of both blood PCSK9 protein and low-density lipoprotein cholesterol (LDL-C) levels of approximately 90% and 60%, respectively, following administration of a single gene editing treatment. These data showed durability out to 10 months at the most recent analysis.
Verve’s gene editing treatments are designed to make a single DNA spelling change in a target gene, permanently turn off a disease-causing gene in the liver, and stop the production of a specific protein known to drive atherosclerotic cardiovascular disease (ASCVD). The company’s initial gene editing programs utilize base editing technology, comprising a messenger RNA encoding for an adenine base editor, as well as a guide RNA packaged in an engineered lipid nanoparticle (LNP). The company’s pipeline is led by VERVE-101, which is designed to permanently turn off the PCSK9 gene in the liver. Inhibition of the PCSK9 gene has been shown in third-party human clinical trials to lower blood PCSK9 protein as well as LDL-C levels and reduce the risk for ASCVD. VERVE-101 is being developed initially for the treatment of patients with heterozygous familial hypercholesterolemia, a potentially fatal genetic heart disease.
“Our goal is to disrupt the cardiovascular disease chronic care model by providing a new therapeutic approach with single-course, in vivo liver-targeted gene editing treatments aimed at addressing the root causes of this highly prevalent and life-threatening disease,” said Sekar Kathiresan, M.D., chief executive officer of Verve. “VERVE-101 brings together multiple breakthroughs in 21st century biomedicine – human genetic analysis, gene editing, mRNA-based therapies and LNP delivery. We believe that these data showed evidence of our ability to precisely, predictably and durably turn off the PCSK9 gene, resulting in measurably lower LDL-C in NHPs. We believe these findings further validate base editing as a potentially viable approach to providing long-term benefit for patients with or at risk for ASCVD.”
The NHP data published in Nature are from short-term studies using VERVE-101 and a long-term study using a VERVE-101 precursor formulation. Key observations in the paper include:
- Optimized VERVE-101 formulation, with substantial potency observed at LNP doses as low as 0.5 mg/kg
- At least 50% average whole liver editing in NHPs, and as high as 76% whole liver editing, across dose levels ranging from 0.5 mg/kg to 3.0 mg/kg, observed at two weeks following a single-dose treatment with VERVE-101
- Durable lowering of blood PCSK9 protein of approximately 90% and LDL-C of approximately 60% observed in a long-term study following a single-dose of a VERVE-101 precursor formulation, with reductions observed to be stable at eight months, and which in the most recent analyses, have been maintained out to 10 months
- VERVE-101 was generally well-tolerated in NHPs, with only mild transient elevations of alanine aminotransferase (ALT) observed, consistent with mild acute liver injury, which was resolved to within the normal range within one week of dosing
- VERVE-101 biodistribution indicated that most editing in NHPs was confined primarily to the liver, with lesser rates of editing observed in the spleen and adrenal glands
In addition, in preclinical studies, there has been no observation of off-target editing with VERVE-101 across any of 63 potential off-target sites identified by the ONE-seq and Digenome-seq techniques, as assessed in primary human hepatocytes.
“We believe that in vivo liver gene editing has the potential to offer meaningful, long-term treatment options for ASCVD, and these data further support that hypothesis,” said Andrew Bellinger, M.D., Ph.D., Verve’s chief scientific officer and chief medical officer, and co-senior author of the Nature publication. “We are encouraged to see high levels of liver editing at LNP dose levels as low as 0.5 mg/kg in NHPs, which we believe may be a powerful predictor of efficacy in humans for gene editing. These data, along with the evidence of favorable tolerability in NHPs and no observed off-target editing in human liver cells, provide rationale to evaluate the utility of the base editing approach in patients with ASCVD.”
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.