Harmony Biosciences is a private pharmaceutical company based in Plymouth Meeting, PA. Established in October 2017, the company’s vision is to develop and provide new medications to help people who are living with rare, neurological diseases.
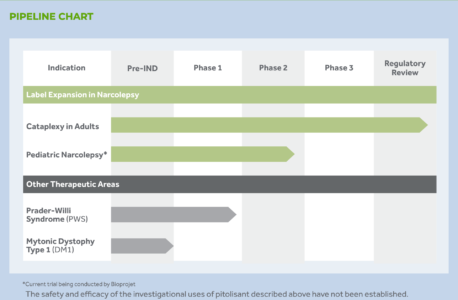
In October 2017, the company acquired exclusive U.S. rights to develop, register and market the drug pitolisant (WAKIX) from French company, Bioprojet SCR (Bioprojet) to address unmet needs for patients with sleep and other central nervous system disorders. pitolisant was approved for the treatment of excessive daytime sleepiness in adult patients with narcolepsy in August 2019. The company expects to initiate a Phase 3 trial for EDS and cataplexy in pediatric patients in the 2H21. It also plans to begin Phase 2 trials for EDS and other key symptoms in patients with Prader-Willi Syndrome in the 2H20 and in adult patients with myotonic dystrophy in the 1H21.
Pipeline
The company is currently evaluating the novel mechanism of action of pitolisant for broader applications in neurological disorders that may be mediated by the H3 receptor. This mechanism-based approach for pitolisant offers the potential for future clinical development in a variety of conditions beyond disorders of wakefulness.
Harmony is actively evaluating the potential addition of new compounds to its portfolio with a focus on orphan/rare neurological disorders.
About Narcolepsy
Narcolepsy is a rare, chronic, debilitating neurologic disorder of sleep-wake state instability that impacts up to 200,000 Americans and is primarily characterized by excessive daytime sleepiness (EDS) and cataplexy – its two cardinal symptoms – along with other manifestations of REM sleep dysregulation, which intrude into wakefulness. EDS is the inability to stay awake and alert during the day and is the symptom that is present in all people living with narcolepsy. In most patients, narcolepsy is caused by the loss of hypocretin, a neuropeptide in the brain that supports sleep-wake state stability. This disorder affects men and women equally, with typical symptom onset in adolescence or young adulthood; however, it can take up to a decade to be properly diagnosed.
Narcolepsy is a rare, chronic, debilitating neurologic disorder of sleep-wake state instability. Narcolepsy impacts up to 200,000 Americans and is primarily characterized by excessive daytime sleepiness, cataplexy, and other manifestations of REM sleep dysregulation, which intrude into wakefulness. In most patients, it is caused by the loss of hypocretin, a neuropeptide in the brain that supports sleep-wake state stability.
This disorder affects men and women equally, with typical symptom onset in adolescence or young adulthood; however, it can take up to a decade to be properly diagnosed. Narcolepsy can cause significant burden on patients and their families, affecting their ability to perform routine tasks, limit achievement at school and work, impact social relationships and cause impairment in overall quality of life.
Patients At The Heart
In April of this year, Harmony announced its Patients at the Heart grant program underscoring the company’s ongoing commitment to support the comprehensive needs of people affected by narcolepsy. The second annual Patients at the Heart grant program was launched with increased funding to further support patient-centric organizations working to address the challenges of people living with narcolepsy during the challenging COVID-19 pandemic. The company’s patient support program will continue to provide medication free of charge for eligible patients who have lost health insurance or are underinsured, while providing “contactless” shipping for home delivery.
Early Launch Metrics
As of June 30, 2020, over 1,750 unique health care professionals (HCPs) (out of a total of approximately 8,000 HCPs who treat approximately 90% of diagnosed narcolepsy patients) have prescribed WAKIX since it became available in November 2019 to a total of over 2,700 unique patients (out of the approximately 42,000 diagnosed and treated narcolepsy patients in the United States). Harmany has secured formulary access for over 166 million lives, which represents 70% of our target covered lives, which the company defines as a group of certain public and private payors that account for approximately 80% of all covered lives in the U.S.
For the three months ended March 31, 2020, net sales of WAKIX were $19.8 million, and for the three months ended June 30, 2020, net sales of WAKIX were $38.0 million.
Investors Prior To IPO
Harmony’s financing has come from equity investors including: Bioprojet; Fidelity Management and Research Company; HBM Healthcare Investments; Nan Fung LIfe Sciences; Novo Holdings; Paragon Biosciences, LLC; Valor Equity Partners; venBio Partners; Vivo Capital; Aisling Capital and Soros Fund Management.
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, warranties or conditions of any kind.

