U.S.-based cancer testing startup Grail Inc has filed for a U.S. initial public offering (IPO) to complete the commercialization of its population-scale multi-cancer early detection testing capability based on a technology called liquid biopsy.
Grail was spun out of gene sequencing company Illumina Inc in 2016, with investments from ARCH Venture Partners, Sutter Hill Ventures and Bezos Expeditions, the venture investment fund of Jeff Bezos.
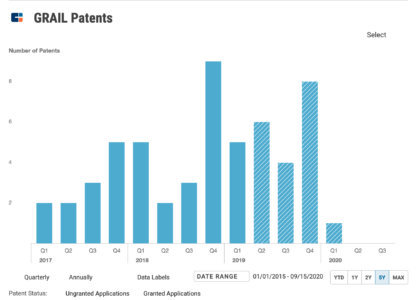
The company set a placeholder amount of $100 million for the IPO, without disclosing the size of its offering. Illumina is the majority shareholder in Grail with a 14.6% stake, according to the company’s filing with U.S. SEC.
At last year’s American Society of Clinical Oncology (ASCO) Annual Meeting, the company presented data from a study, the Circulating Cell-free Genome Atlas (CCGA) Study. In the largest study of its kind, Grail reported conclusions from the CCGA demonstrating that a single blood test can detect more than 50 cancers. This study encapsulates the promise of cancer control through early detection. Here are more details reported by Annals of Oncology.
Grail’s liquid biopsy tool is called Galleri. The company claims that data in a clinical study showed that when Galleri detected a cancer, it was also able to localize the cancer signal with high accuracy. In the second sub-study (CCGA-2) of the CCGA, when a cancer signal was detected, an earlier version of Galleri localized the cancer signal in 96% of the samples, and of these, Galleri correctly localized the cancer signal in 93%. Early data also suggested that indolent cancers are unlikely to be detected by Galleri, potentially reducing the problem of treating over-diagnosed cancers.

Serendipity motivated Grail’s creation as a separate entity. Illumina was developing a cell-free nucleic acid (cfDNA) test in a non-invasive prenatal setting that detected fragmentary DNA for signals of fetal chromosomal abnormalities. After 100,000 prenatal tests, about 20 were anomalous. Illumina dug in on the anomalous DNA samples and discovered late-stage, undiagnosed cancer.
Today, there are only five cancer screening tools recommended by the U.S. Preventive Services Task Force. They are: breast, lung, cervical, prostate and colorectal. According to the U.S. Centers for Disease Control and Prevention (CDC), other types of cancer screenings, “have not been shown to reduce deaths from those cancers.” These recommended secondary prevention tests are imperfect in terms of specificity (low false positive) and sensitivity (detection), yet they remain the standard of care. Despite the known benefits of training drugs to target specific genetic features (mutations), the same potential has not been adopted in diagnostics. In fact, there has been relatively little research and funding into cancer prevention. The reasons for this include government central planning combined with bureaucratic skepticism around the efficiency of clinical testing and prevention measures and concerns about over-diagnoses and over-treatment.
In an interview with Ark Invest, Dr Dr. Bert Vogelstein, Director of the Ludwig Center for Cancer Genetics and Therapeutics at the Kimmel Comprehensive Cancer Center within the Johns Hopkins University School of Medicine, says, “Why do I say we have under-diagnosis? Because there are half a million people who die in this country each year from cancer. Almost all of them die only because their cancers weren’t detected until too late.”
Disease begins before diagnosis, yet there is no feedback loop in the clinical setting to track misdiagnosis, diagnostic inaccuracies or systemic bias. I can only conclude, based on the absence of measurement standards, that the healthcare industry believes it is immune from such maladies. The centrally-planned regulations for electronic health records (EHR) were designed mainly for clinical tracking of billing codes, not for patient portability, quality control or feedback. If creativity is novelty plus value, the healthcare industry too often destroys both. Liquid biopsy offers the brightest hope to drive novelty and much-needed price deflation to the heart of the industry.
Yoking your total addressable market (TAM) to the delta of five screening tools going to 50 is a king-making leap. Grail’s own expression of TAM is based on a 2006 article by Kevin M. Murphy and Robert H. Topel published in the Journal of Political Economy and the U.S. Bureau of Labor Statistics inflation calculator. The company points to a metric (1% reduction in cancer mortality in the U.S.) that translates into $695 billion in today’s dollars from increased quality of life, productivity and survival. This estimate does not include intangible benefits such as the decreased emotional burden to family, friends and caregivers. That’s a big TAM for a company with zero revenue, accumulated deficit of $1.4 billion and $2 billion of funding to date.
While the company waits for various government approvals (ie. medicare reimbursement), it intends to pursue an initial market representing a significant segment of the overall early detection market of 107 million U.S. individuals between the ages of 50-79, the cohort most vulnerable to cancer and (I would argue) most sensitive to the risks of relying on a centrally-planned government healthcare policy. The channels for this initial market include physician-directed channels (concierge practices and executive health programs); large, self-insured employers; and progressive, integrated health systems.
Galleri’s planned 2021 launch is tied to additional validation work underway through Grail’s 6,200-person, clinical implementation study called Pathfinder. This study is being conducted under an investigational device exemption (IDE) to evaluate GRAIL’s multi-cancer early detection test. The company believes that its IDE application is the first approved by the Food and Drug Administration (FDA) for a return-of-results study involving a multi-cancer next-generation sequencing-based blood test.
One area not mentioned in Grail’s documents involves companion diagnostics, an emerging diagnostic tool that is under the liquid biopsy umbrella. Last month, the U.S. FDA approved the first liquid biopsy companion diagnostic that also uses next-generation sequencing (NGS) technology to identify patients with specific types of mutations of the epidermal growth factor receptor (EGFR) gene in a deadly form of metastatic non-small cell lung cancer (NSCLC). This is the first approval to combine two technologies — NGS and liquid biopsy — in one diagnostic test in order to guide treatment decisions. That approval went to Guardant Health, a competitor.
“We are a healthcare company,” appears in the first lines of Grail’s 200+ page regulatory filing with the SEC. After reading most of the document, I can’t escape these words because healthcare in the U.S. so often means absence of innovation. The bulk of Grail’s revenues will derive from the government, and with that strategy comes the (potential) tyranny of developing therapies based on targeting government-mandated medical billing codes as a prime directive, not patient health outcomes. Nevertheless, the cost curve the company is riding is the ever-declining cost of DNA sequencing, so it is possible for liquid biopsy technology to achieve population-scale improvement in health outcomes. For now, Grail is on track to get there.
There are many companies innovating in the liquid biopsy space, propelled by an accelerating pace of discovery in cancer biology (e.g., methylation and cfDNA) rooted in machine-learned bioinformatics and high-intensity sequencing. One in particular stands out for its understanding of increasing returns to scale and the power of innovation where software is applied to previously analog problems. Quantgene is a good example of a company thinking through the lens of value- or outcome-based solutions. It has the commercial instincts to hunt down revenue along the path to developing a direct-to-consumer, recurring revenue product based on a constellation of tests including liquid biopsies. And its business strategy aligns patients interests with its corporate objectives.
In a recent interview with Synthetic.com, Quangene CEO, Jo Bhadki, discussed how his company exploited the COVID pandemic by tooling up the most accurate and fastest COVID PCR diagnostic test in the country. Within three weeks of redeploying some of the company’s liquid biopsy assets, the team had spun up a business with more than $1m in revenue for the three week period. The companies served by a highly reliable COVID test are then aware of Quantgene’s capabilities which can lead to a larger relationship on the liquid biopsy side of the business. It’s this scrappiness quality I could not find in Grail’s documents, but the industry is likely to be so deep and wide, there is room for a variety of business models to succeed.
Update September 21: Illumina decided to acquire Grail for $8 billion. The IPO is cancelled.
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, warranties or conditions of any kind.

