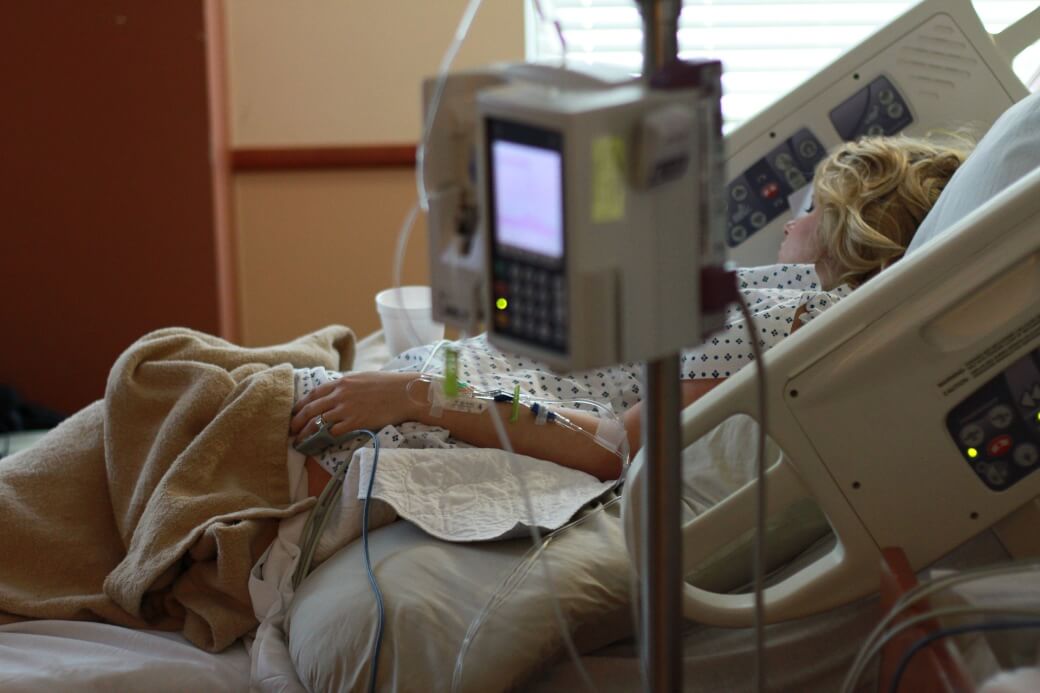
Admetsys is developing what can be described as an artificial pancreas for patients requiring precise glucose control in a hospital setting.
I spoke with Jeff Valk, CEO, who aptly walked me through his company’s technology and its vital importance for, “The most metabolically variable humans on earth.” To put at-risk patients on a solid ground (metabolically speaking), what’s needed is continuous real-time monitoring and dosing.

The Problem
Glucose control (hypo- and hyperglycemia) in seriously ill patients is a major problem for hospitals in terms of time, money and risk. For example, hyperglycemia is a frequent consequence of severe illness, occurring in both diabetic and non-diabetic patients, due to altered metabolic and hormonal systems, impaired gastrointestinal motility, altered cardiac function, increased catecholamine production, altered hepatic gluconeogenesis, relative insulin resistance and increased corticosteroid levels.
“Medical studies have established a direct correlation between morbidity and mortality in intensive care patients and the degree of glucose control,” says Mr. Valk.
According to Mr. Valk, the burden is on nurses to monitor patient glucose levels every 1 to 1.5 hours. In addition, nurses must implement increasingly complex procedures to monitor and control patients’ glucose levels. This level of attention by healthcare professionals is not practical for busy hospital intensive care units. Furthermore, as a result of increases in medical malpractice claims, stricter control regimens have been imposed on hospital staffs. These control regimens are often complex and increase the already heavy burden on health care professionals.
The Solution
Admetsys has developed a system that develops of novel “Model of Human Matabolism” that manages blood glucose. A standard IV line containing a tiny laboratory-on-a-chip gets embedded within each patient’s IV. The device linedraws a small blood sample (less than a mL) every few minutes. It measures glucose electrochemically and returns the blood back to the patient. The system constructs a real-time computational model using a patient-specific learning algorithm and then precisely optimizes a balance between insulin and glucose, delivered through high precision syringe pumps. No manual intervention is required after a patient is being treated with the Admetsys device. Cost savings run in the thousands of dollars per patient. According to Mr. Valk, clinical trials show excellent results:
– 2.5 hour mean time to normoglycemia (80-125mg/dL)
– 97.6% normoglycemic control (80-125mg/dL)
– Zero incidents of hypoglycemia
The Boston-based company is nearing the regulatory approval stage for its product that, according to Mr. Valk, is a the result of a father-son collaboration about solving a major problem for hospitalized patients. Remarkably, the company has relied mostly on self-funding to date.
There are three recently-completed Food and Drug Administration (FDA) clinical research studies. In the EU, a Denmark study is complete, and an EU “CE marking” is expected in the near future. In terms of IP, there are six approved patents, filed internationally, and several more in the application stage.
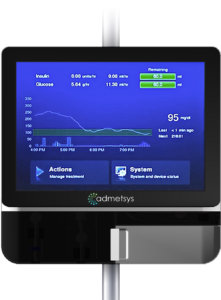
Admetsys has not missed the attention of some large medical device companies. According to Mr. Valk, there are partnerships with Pfizer, Lily and BBraun. And there are other promising patient metabolic factors on the horizon that the company’s technology may provide similar benefit for.
I asked Mr. Valk about the potential for an at-home device at some point in the future. He wasn’t willing to say for certain whether the consumer market held any potential, but with the recent acceleration of telemedicine in response to COVID-19, I think we may be hearing about this dimension at some point soon.