There are 37 trillion or so cells in our bodies that work together to give us life. But it may surprise you that we still haven’t put a good number on how many distinct cell types there are within those trillions of cells.
That’s why in 2016 a team of researchers from around the globe launched a historic project called the Human Cell Atlas (HCA) consortium to identify and define the hundreds of presumed distinct cell types in our bodies. Knowing where each cell type resides in the body, and which genes each one turns on or off to create its own unique molecular identity, will revolutionize our studies of human biology and medicine across the board.
Since its launch, the HCA has progressed rapidly. In fact, it has already reached an important milestone with the recent publication in the journal Science of four studies that, together, comprise the first multi-tissue drafts of the human cell atlas. This draft, based on analyses of millions of cells, defines more than 500 different cell types in more than 30 human tissues. A second draft, with even finer definition, is already in the works.
Making the HCA possible are recent technological advances in RNA sequencing. Researchers use it to detect and analyze all the messenger RNA (mRNA) molecules in a biological sample, in this case individual human cells from a wide range of tissues, organs, and individuals who voluntarily donated their tissues.
Cell Therapies
In the U.S., the Center for Biologics Evaluation and Research (CBER) regulates cellular therapy products, human gene therapy products, and certain devices related to cell and gene therapy. CBER uses both the Public Health Service Act and the Federal Food Drug and Cosmetic Act as enabling statutes for oversight.
Cellular therapy products include cellular immunotherapies, cancer vaccines, and other types of both autologous and allogeneic cells for certain therapeutic indications, including hematopoetic stem cells and adult and embryonic stem cells. Human gene therapy seeks to modify or manipulate the expression of a gene or to alter the biological properties of living cells for therapeutic use. CBER has approved both cellular and gene therapy products – a list of these products may be found here.
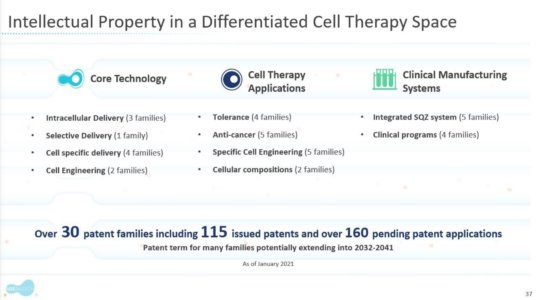
SQZ Biotechnologies (Ticker: SQZ) is a clinical-stage biotechnology based in Watertown, MA developing novel cell therapies for indications in oncology, infectious disease, and immune tolerance. SQZ ‘s pipeline is based on their proprietary cell squeeze technology, an innovative method to manufacture next-generation cell therapies. Lead asset SQZ-PBMC-HPV is currently in a phase 1/2 trial in HPV+ solid tumors. Pipeline assets nearing the clinic are pursuing opportunities in oncology and infectious disease. The company’s technology is simple and appears easy to scale.
Under SQZ’s 2018 Roche agreement ($3 billion in total potential value), there is potential for $217m in development milestone payments. Of that, under SQZ’s leading APC (antigen presenting cell) therapy currently in Phase 1/2 clinical trials, there are “specified incremental payments of between $15m to $50m” depending on undisclosed quality/quantity of clinical results. Such results are expected in 2H22.
Recent highlights:
SQZ ® Antigen Presenting Cell (“APC”) Platform in Oncology
- Granted FDA Fast Track Designation for SQZ-PBMC-HPV, our APC clinical candidate, for HPV16+ advanced or metastatic tumor;
- Continued enrollment of high dose monotherapy and combination with checkpoint inhibitors in the Phase 1/2 (SQZ-PBMC-HPV) trial;
SQZ ® Enhanced Antigen Presenting Cell (“eAPC”) Platform in Oncology
- Presented SQZ® eAPC preclinical data at the American Association for Cancer Research (AACR) annual meeting demonstrating that delivery of multiple mRNAs encoding for disease-specific antigens and immune stimulators had a synergistic effect that substantially increased killer T cell activity in humanized mouse models
- Initiated enrollment and opened additional sites for the monotherapy stage of the COMMANDER-001 Phase 1/2 (SQZ-eAPC-HPV) trial;
SQZ ® Activating Antigen Carriers (“AAC”) Platform in Oncology
- Published trial in progress poster at the AACR annual meeting highlighting the SQZ® AAC platforms potential to drive robust CD8 T cell activation and tumor killing;
- Continued enrollment and opened additional sites for the monotherapy stage of the ENVOY-001 Phase 1/2 (SQZ-AAC-HPV-101) trial;
SQZ ® Tolerizing Antigen Carriers (“TAC”) Platform in Immune Tolerance
- Published comprehensive preclinical research in Frontiers in Immunology Progressed studies supporting anticipated TAC IND submission for celiac disease in the first half of 2023; company’s POC manufacturing system intended to produce clinical batche;
SQZ ® Point-of-Care Manufacturing
- Presented non-clinical POC manufacturing performance data at the American Society for Gene and Cell Therapy (ASGCT) annual meeting demonstrating reduced manufacturing time and comparable or improved product specifications relative to current cleanroom-based processes;
As of June 30, 2022, SQZ had cash and cash equivalents of $105.6 million and reported that it anticipates this will be sufficient to fund operating expenses and capital expenditure requirements into the fourth quarter of 2023. It’s current market cap is $94 million.
Founded in 2015 after more than a decade of research, ProKidney (Ticker: PROK) is an early leader in the treatment of chronic kidney disease (CKD). The company’s lead product candidate, REACT™ (Renal Autologous Cell Therapy) is a first-of-its-kind disease modifying cell therapy made from a patient’s own renal cells. REACT™ has the potential to not only slow and stabilize the progression of CKD, but in some cases drive meaningful improvement in kidney function, as measured by estimated glomerular filtration rate (eGFR). REACT™ has been studied in multiple clinical trials to-date, and a Phase 3 registrational program was initiated in January 2022. REACT™ received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA in 2021.
In 2018, total U.S. annual spend (including private payers) on CKD and end-stage renal disease (“ESRD”) was up to $500B Medicare costs alone for CKD. ESRD patients were $130B Medicare spend on dialysis costs an average of nearly $100K per patient per year over the lifetime of the patient (average term for dialysis is 5 years). For private payers, CKD medical costs and dialysis can be up to 4x the Medicare cost. Initial REACT® target market: 4M to 5M diabetic patients with CKD stages 3a, 3b, and 4 at very high risk of renal failure with severe albuminuria and eGFR’s between 20 – 50 ml/min/1.73m2.
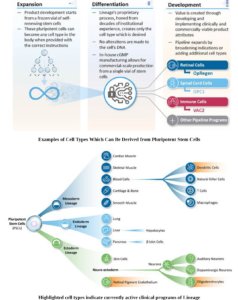
Lineage’s (Ticker: LCTX) stated goal is to address unmet medical needs by developing and advancing allogeneic, or “off-the-shelf,” treatments comprised of functional cells derived by differentiation of pluripotent cells from established and self-renewing cell lines. We direct pluripotent cells to become specific cell types and use those differentiated cells as treatments to restore diseased or diminished functions, such as impaired vision, loss of movement and sensation, or to increase immune response to tumors or infectious agents.
The company’s cash and cash equivalents totaled $78.1 million as of March 31, 2022 with a annual burn rate of approximately $20 million. Current market cap: $226 million
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.