In biotech investing, distilling exceptional opportunities from the average (or worse) ventures requires a keen eye on insider activities, financial health, and clinical progress. Beam Therapeutics (BEAM) presents a case that demands caution, with insider stock sales, a substantial accumulated deficit, and limited clinical advancements raising significant concerns. In contrast, Intensity Therapeutics (INTS) is an example of prudent management and efficient capital use, making noteworthy clinical strides with far fewer resources and zero insiders cashing out.
While a direct comparison of the two companies has little meaning, comparing the conduct of the CEOs and the other insiders of the companies is a useful exercise for potential investors. Combining knowledge of insider selling with other research is the best approach for developing a more informed picture of risks and opportunities.
One striking example of a biotech company infested with insider selling is Invitae Corp. At one point, Invitae was valued at approximately $13 billion, with expectations built around the company’s potential in genetic testing, particularly during the pandemic. However, the company’s actual performance, characterized by significant losses and a lack of distinctive products, led to a reassessment of its value and growth prospects. The market’s initial enthusiasm did not align with the company’s financial realities, contributing to its challenges. The company entered bankruptcy in 2024. How could an investor have better judged the risks inside this failing company? Observe the following:
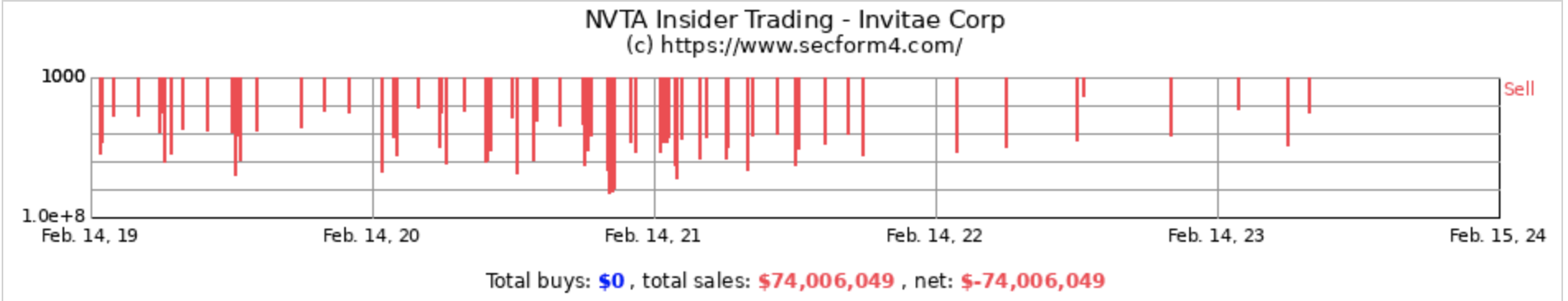
Insider Sales: A Red Flag at Beam
Consider this statement made by BEAM’s CEO, John Evans, in 2023: “We really think of this as a very attractive platform. That’s why we’re investing so aggressively in it because we think that the long-term productivity of it and frankly again the impact we can have on many patients is really there.” According to records filed with the SEC, John Evans has sold more than $25 million of stock during his tenure, in addition to his annual cash compensation of approximately $1 million, half of which is described by the company as a “bonus.” The truth is that Mr. Evans has invented nothing, risked nothing and invested nothing and has yet to guide BEAM to the simple milestone of dosing a single patient in a Phase 1 trial, as of December 31, 2023. In contrast, INTS dosed over 200 patients before it IPO’d, and many of those patients received actual clinical benefit.
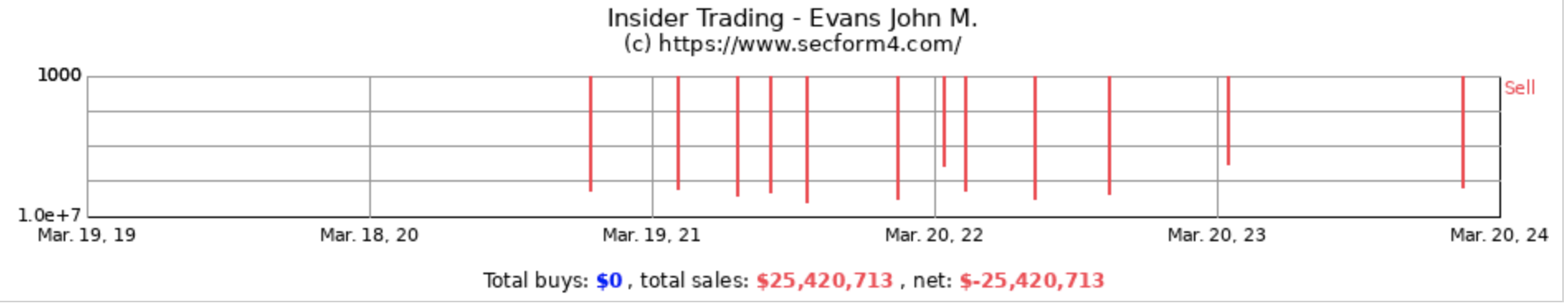
BEAM’s pattern of insider stock sales emerges as a critical red flag, suggesting a lack of confidence in the company’s future among those who know it best. Such activity, particularly against the backdrop of the company’s slow progress in groundbreaking yet unproven gene editing technology, heightens the investment risk. The biotech sector’s inherent uncertainties make insider conviction a key part of risk assessment.
In fact, all the senior executives have been dumping the company’s stock in recent years without a single instance of an open market cash purchase even after the company’s stock declined by more than 50% last year.
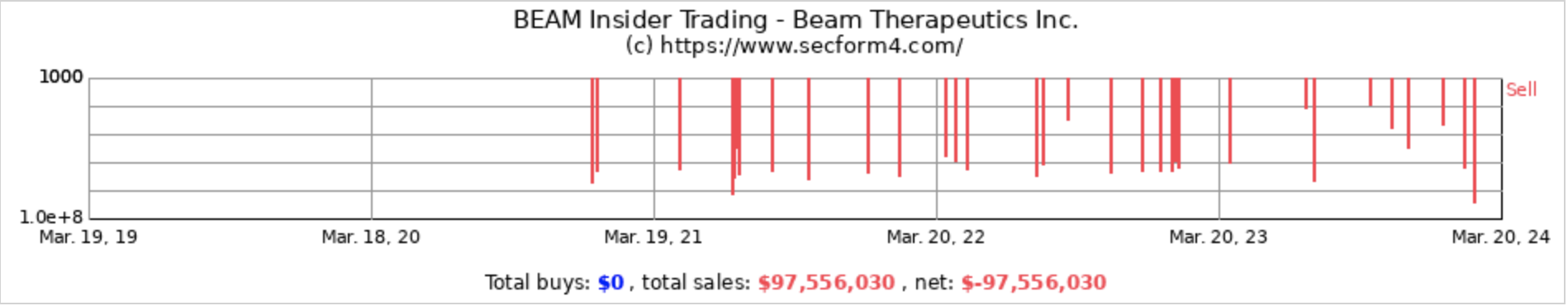
From December 31, 2020, to December 31, 2023, the total number of common shares surged from 57,254,178 to 81,632,496—a 43% increase in shares, reflecting significant dilution borne by shareholders. Mr. Evans is earnestly urging these same shareholders to maintain their long-term faith in the company all the while he and his team are dumping nearly $100 million of shares on the open market. What could CEO Evans possibly need with $25 million in addition to his $1 million cash salary?
Intensity’s Contrasting Approach
INTS, under the stewardship of CEO and founder Lew Bender, provides a stark contrast. Bender’s commitment, demonstrated through his personal investment and leadership, from inception to Phase 3 clinical trials, highlights a strategic and efficient approach to biotech development. This hands-on leadership and judicious use of capital, without the shadow of insider sales, underscore a company moving confidently toward its goals. There has not been a single stock sale recorded from an insider with the SEC since the company went public in 2023. Update: Lew reminded me he invested $100,000 into the IPO even though he already owned 2,000,000 shares.
Bender’s Vision and Leadership
Lew Bender’s personal investment along the support from a select group of private investors have been pivotal in INTS’s focused and agile development strategy in its early years. Achieving significant clinical milestones using his own intellectual property and funds along with limited external funding (pre-IPO) not only showcases Bender’s strategic acumen but also preserves the company’s equity value, aligning with long-term shareholder interests. This approach, contrasting with BEAM’s challenges, emphasizes the importance of leadership commitment and strategic resource allocation in the biotech industry.
The following is an example of stellar clinical progress, deploying approximately $53 million of funds (or about 5% of the amount deployed by BEAM), as of December 31, 2023:
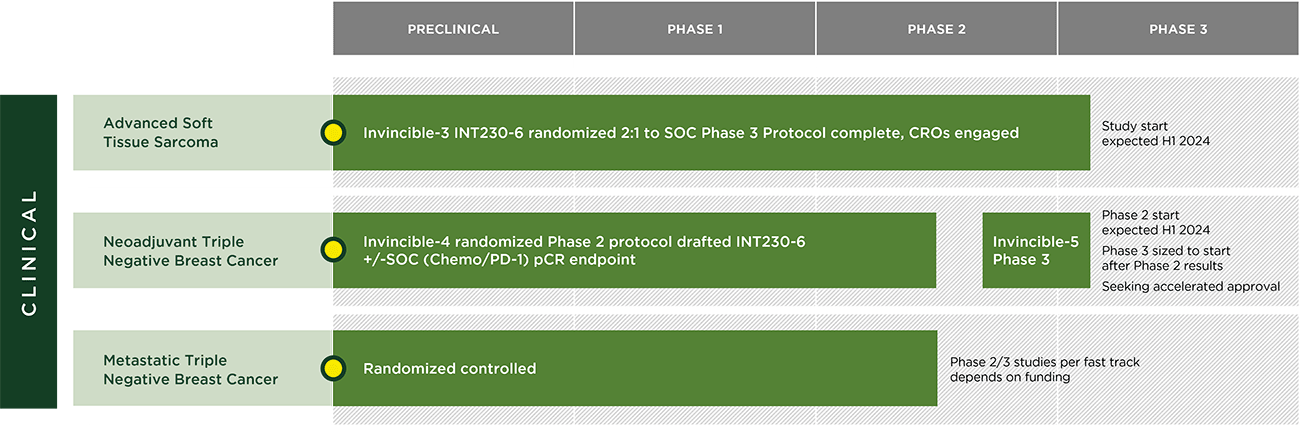
INTS’s pipeline is focused on realizing the full potential of INT230-6 in metastatic and local disease settings to help cancer patients with major unmet medical need. The company is exploring the use of INT230-6 across multiple cancer types (including those types that do not normally respond to immunotherapy) and “hot” tumors (cancer types that are more likely to respond to immunotherapy).
Based on data generated in IT-01 and the INVINCIBLE-2 studies, the company’s current forward pipeline consists of:
- A Phase 3 open-label, randomized study testing the superiority INT230-6 used as monotherapy compared to the standard of care drugs in 2nd and 3rd line treatment for certain soft tissue sarcoma subtypes. For every three patients enrolled, two will receive INT230-6 and one will receive SOC drug(s) chosen by the investigators depending on the type of sarcoma. The company is working with several contracted vendors to initiate the Phase 3 trial. INTS plans to enroll 333 patients with an endpoint of overall survival. In September 2023, INTS announced that the FDA granted orphan drug designation for the treatment of soft tissue sarcoma to the three active moieties comprising INT230-6: cisplatin, vinblastine sulfate, and the diffusion enhancer SHAO;
- A Phase 2/3 study testing INT230-6 in combination with the SOC treatment (chemotherapy/immunotherapy) compared to the SOC alone in women with triple negative breast cancer in presurgical (neoadjuvant) breast cancer. The endpoint for the Phase 2 portion of the trial is the change in the pathological complete response rate for the combination compared to the SOC alone.
- A Phase 2/3 clinical study in metastatic triple negative breast cancer, contingent on raising additional capital to fund the study. In 2018, INTS received Fast Track Designation by the FDA to use INT230-6 in metastatic triple negative breast cancer for patients whose cancer has progressed following one or two prior drug treatments.
The ideas presented on this site do not constitute a recommendation to buy or sell any security. Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action. You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SYNTHETIC.COM nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance. SYNTHETIC.COM posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied. The material on this website is provided for information purpose only. SYNTHETIC.COM does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, recommendations, warranties or conditions of any kind.

