ArcherDX, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its next-generation sequencing (NGS) device under development as a companion diagnostic (CDx) to detect neurotrophic receptor tyrosine kinase (NTRK) gene fusions that lead to the formation and growth of cancer. ArcherDX’s NGS technology utilizes proprietary Anchored Multiplex PCR (AMP
NTRK fusions are rare, medically-actionable mutations common in some pediatric cancers.
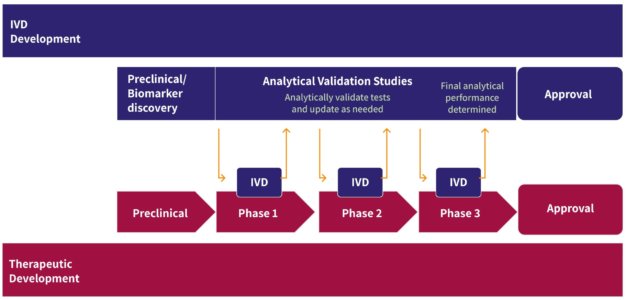
ArcherDX is developing and pursuing regulatory clearances for its NGS diagnostic to help solve for the underutilization of targeted therapies. ArcherDX’s technology is designed to enable the use of the diagnostic in community and regional settings where the lack of infrastructure and expertise to implement sophisticated genomic analysis can limit a clinician’s ability to optimize therapy for patients through diagnosis, prognosis and therapy selection.
“NTRK gene fusions are notoriously difficult to detect in cancer.i ArcherDX’s next-generation sequencing technology uses RNA to provide sensitive NTRK fusion detection without requiring prior knowledge of fusion partners for any solid tumor,i“ said Jason Myers, Ph.D., Chief Executive Officer and co-founder of ArcherDX. “Our goal is to develop products to help clinicians receive the right information at the right time to make the right treatment decision.”
ArcherDX recently announced a collaboration to develop an NGS-based CDx for a TRK inhibitor.
The Breakthrough Devices Program is a voluntary program intended to expedite the review, development and assessment of certain medical devices that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions for which no approved or cleared treatment exists or that offer significant advantages over existing approved or cleared alternatives.